You are here
U.S. FDA panel narrowly backs Pfizer RSV vaccine for older adults
Primary tabs
Wed, 2023-03-01 09:50 — mike kraft
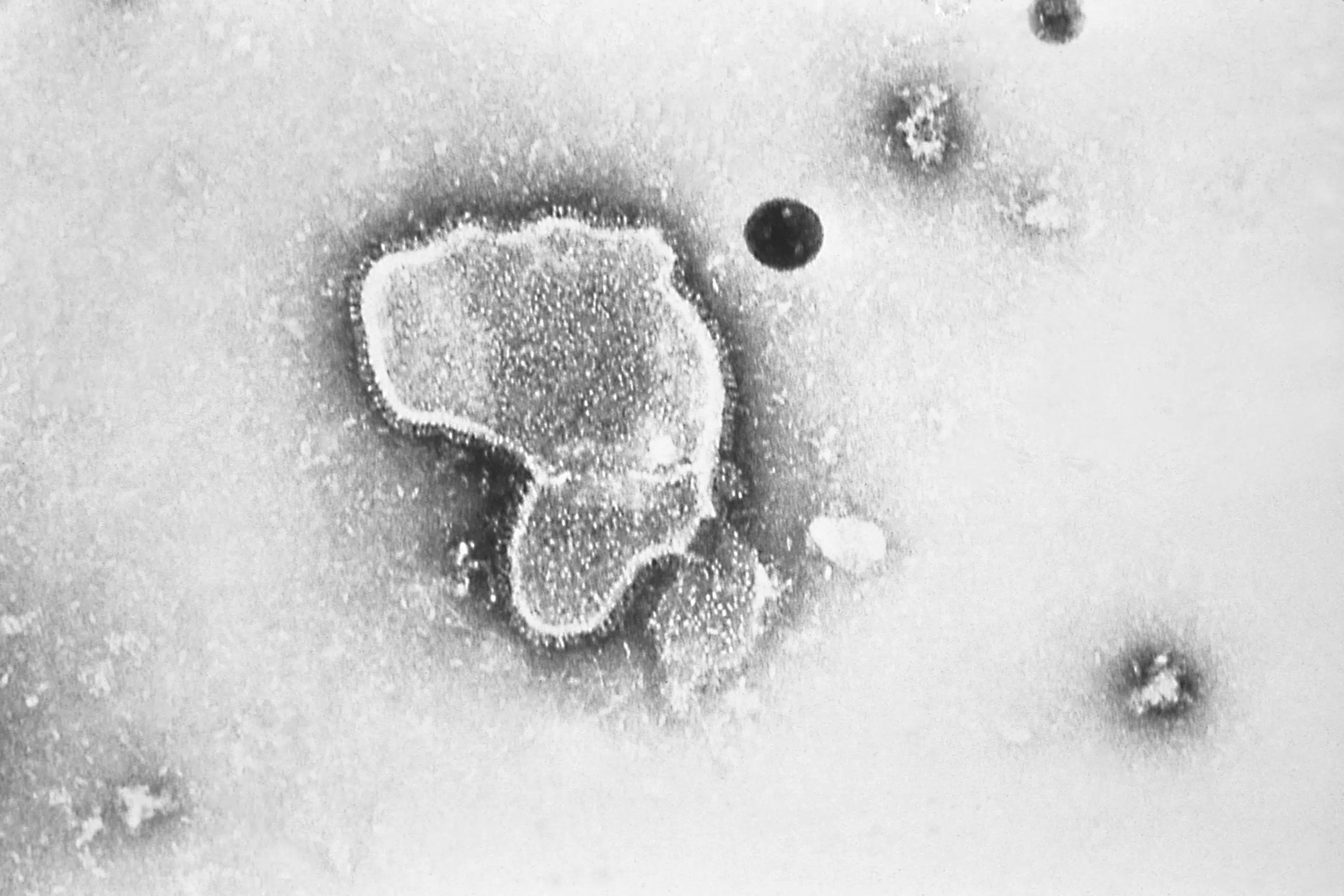 FDA panel narrowly backs Pfizer RSV vaccine for older adults WASHINGTON (AP) — Federal health advisers on Tuesday narrowly backed an experimental vaccine from Pfizer that could soon become the first shot to protect older adults against the respiratory illness known as RSV. AP NEWS
FDA panel narrowly backs Pfizer RSV vaccine for older adults WASHINGTON (AP) — Federal health advisers on Tuesday narrowly backed an experimental vaccine from Pfizer that could soon become the first shot to protect older adults against the respiratory illness known as RSV. AP NEWS
 FDA panel narrowly backs Pfizer RSV vaccine for older adults WASHINGTON (AP) — Federal health advisers on Tuesday narrowly backed an experimental vaccine from Pfizer that could soon become the first shot to protect older adults against the respiratory illness known as RSV. AP NEWS
FDA panel narrowly backs Pfizer RSV vaccine for older adults WASHINGTON (AP) — Federal health advisers on Tuesday narrowly backed an experimental vaccine from Pfizer that could soon become the first shot to protect older adults against the respiratory illness known as RSV. AP NEWS ...
The Food and Drug Administration panel voted 7-4 on two separate questions of whether Pfizer’s data showed the vaccine was safe and effective against the respiratory virus for people 60 and older. One panelist abstained from voting. The recommendation is non-binding and the FDA will make its own decision on the vaccine in the coming months.
...
Country / Region Tags:
General Topic Tags:
Problem, Solution, SitRep, or ?:
Groups this Group Post belongs to:



Recent Comments