REUTERS by Kate Keller and Ben Hirschler June 17, 2015
LONDON --Drugmakers' plans to conduct vast clinical trials to test and hopefully validate the first Ebola vaccines have been thwarted by success in beating back the deadly epidemic in West Africa.
GlaxoSmithKline, Merck and Johnson & Johnson are struggling to recruit volunteers with enough exposure to the disease to prove whether their vaccines are doing the job and preventing infection.
The story might have been very different with just another three or four months of disease spread, underscoring the need to act more quickly to develop vaccines for emerging diseases....
Guinea, where Ebola is still infecting new victims, as "the only hope" for showing efficacy, according to Kieny and to Adrian Hill, director of the Jenner Institute at Britain's Oxford University.
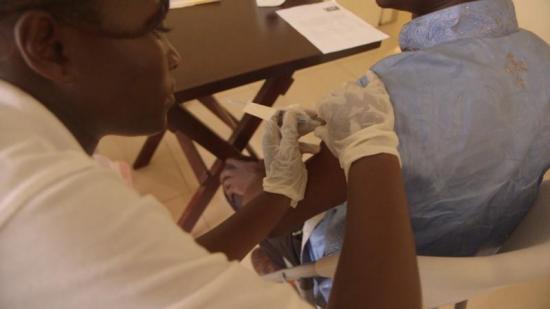
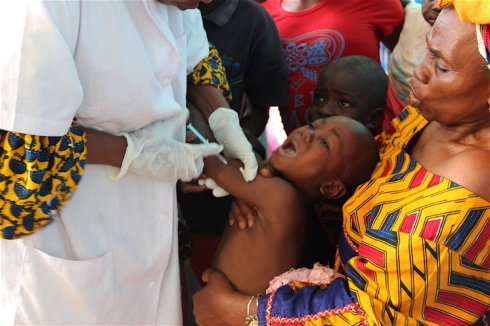
The WHO is overseeing the so-called ring vaccination study in Guinea in which close contacts and family around each new case of Ebola are vaccinated -- either immediately or after a three-week delay -- to see if the shot offers protection.
Read complete story.





Recent Comments