You are here
AstraZeneca's coronavirus vaccine becomes third to begin Phase three trials in the United States
Primary tabs
Tue, 2020-09-01 10:05 — mike kraft
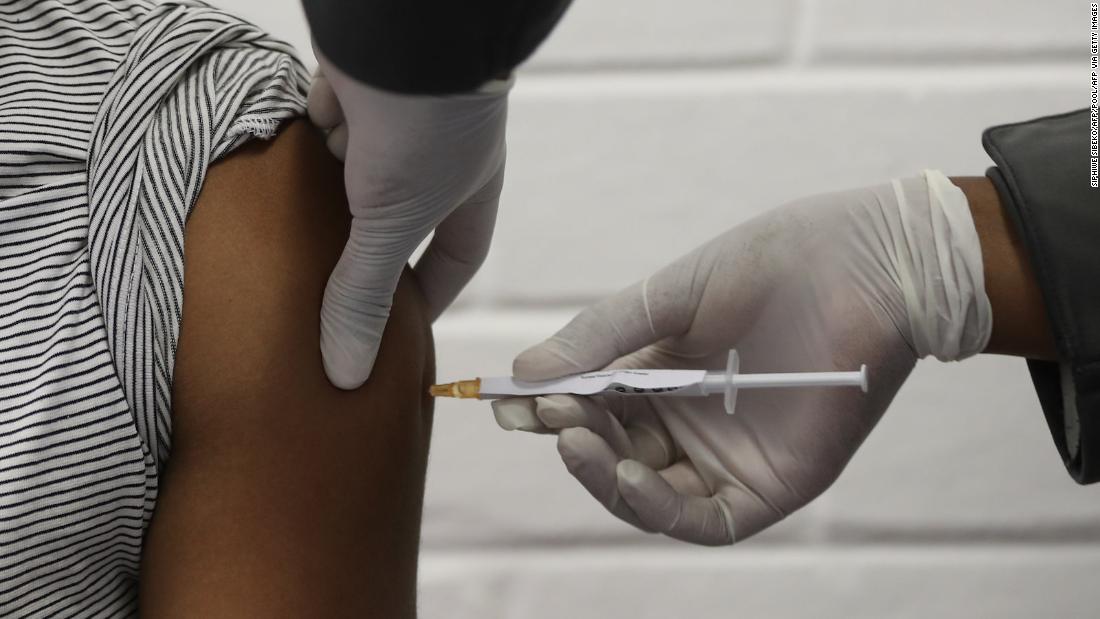 AstraZeneca's coronavirus vaccine becomes third to begin Phase three trials in the United States British drugmaker AstraZeneca says it has started Phase 3 trials of its coronavirus vaccine candidate in the United States. The vaccine, AZD 1222, is the third to enter large-scale trials in the United States, after vaccines made by Moderna and Pfizer/BioNTech. CNN
AstraZeneca's coronavirus vaccine becomes third to begin Phase three trials in the United States British drugmaker AstraZeneca says it has started Phase 3 trials of its coronavirus vaccine candidate in the United States. The vaccine, AZD 1222, is the third to enter large-scale trials in the United States, after vaccines made by Moderna and Pfizer/BioNTech. CNN
 AstraZeneca's coronavirus vaccine becomes third to begin Phase three trials in the United States British drugmaker AstraZeneca says it has started Phase 3 trials of its coronavirus vaccine candidate in the United States. The vaccine, AZD 1222, is the third to enter large-scale trials in the United States, after vaccines made by Moderna and Pfizer/BioNTech. CNN
AstraZeneca's coronavirus vaccine becomes third to begin Phase three trials in the United States British drugmaker AstraZeneca says it has started Phase 3 trials of its coronavirus vaccine candidate in the United States. The vaccine, AZD 1222, is the third to enter large-scale trials in the United States, after vaccines made by Moderna and Pfizer/BioNTech. CNN (CNN)British drugmaker AstraZeneca said Monday it has started Phase 3 trials of its experimental coronavirus vaccine in the United States, becoming the third company to start late-stage trials of a vaccine to prevent Covid-19.
The vaccine, developed in partnership with Oxford University, has the backing of the US federal government. Rivals Moderna and Pfizer/BioNTec already have Phase 3 trials under way, also with federal government funding.
AstraZeneca said it is "recruiting up to 30,000 adults aged 18 years or over from diverse racial, ethnic and geographic groups who are healthy or have stable underlying medical conditions, including those living with HIV, and who are at increased risk of infection from the SARS-CoV-2 virus." ...
The US trial is funded by the federal government's Biomedical Advanced Development Authority and the National Institute of Allergy and Infectious Diseases, part of the National Institutes of Health.
"NIH is committed to supporting several Phase 3 vaccine trials to increase the odds that one or more will be effective in preventing COVID-19 and put us on the road to recovery from this devastating pandemic," said NIH director Dr. Francis Collins said in a statement. "We also know that preventing this disease could require multiple vaccines and we're investing in those that we believe have the greatest potential for success. ...
Both Moderna and Pfizer/BioNTech are still in the process of enrolling their stated goals of 30,000 volunteers each.
Country / Region Tags:
Problem, Solution, SitRep, or ?:
Groups this Group Post belongs to:



Recent Comments