You are here
FIERCE DIAGNOSTICS Oct. 23, 2014
French scientists are developing a diagnostic tool that works similar to a home pregnancy test and can quickly identify the virus through a tiny fluid sample.
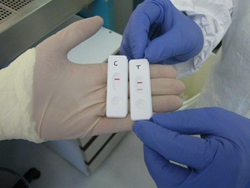
CEA's Ebola testing kit uses strips to rapidly identify the presence of the virus in fluid samples.--Courtesy of France's Atomic Energy Commission
France's Atomic Energy Commission (CEA) is teaming up with European pharma company Vedalab to roll out a user-friendly testing system than could diagnose Ebola in less than 15 minutes, the agency said in a statement. The kit, dubbed "Ebola eZYSCREEN," includes a hand-held device that reads small samples of blood, plasma or urine to detect the virus, and shows results in stripes through a window on the tool.
Unlike other Ebola kits, CEA's test can be used in the field and does not require any additional lab equipment. The agency validated its diagnostic technology earlier this month at a high security lab in Lyon, France, and plans to kick off clinical trials of its prototype kits in Ebola-stricken countries by the end of October.
Read full article
Direct link - French - Vedalab
http://www.vedalab.com/SITE_VEDALAB_WEB/FR/default.awp



Comments
FDA issues emergency authorization for new American Ebola tests
REUTERS OCT. 25, 2014
By Yasmeen Abutaleb
WASHINGTON --U.S. Federal health regulators have granted emergency authorization for two new tests made by BioFire Defense that can detect Ebola in humans more quickly and in hospitals instead of special labs.
The U.S. Food and Drug Administration has been working closely with Salt Lake City-based BioFire, a subsidiary of medical diagnostics maker BioMerieux, to obtain the necessary performance data to allow for the authorizations, the federal agency said Sturday in a news release.
BioFire's tests can detect Ebola in a blood or urine sample in one hour, compared with the 24 to 48 hours current tests take to deliver results, said Matt Scullion, vice president of sales and marketing for BioFire Defense. The test can also be performed in a hospital with BioFire lab equipment, whereas current tests need to be sent to specialized labs.
Read full article
http://www.reuters.com/article/2014/10/25/health-ebola-usa-fda-idUSL2N0SK0US20141025